Trusted shipping
Easy returns
Secure shopping
Buy EverlyWell COVID-19 Test Home Collection Kit - 3pc in United States - Cartnear.com

EverlyWell COVID-19 Test Home Collection Kit - 3pc
CTNR609159 0851044008428 CTNR609159EverlyWell
2027-03-04
/itm/everlywell-covid-19-test-home-collection-kit-3pc-609159
USD
116.15
$ 116 $ 121 4% Off
Item Added to Cart
customer
*Product availability is subject to suppliers inventory
SHIPPING ALL OVER UNITED STATES
100% MONEY BACK GUARANTEE
EASY 30 DAYSRETURNS & REFUNDS
24/7 CUSTOMER SUPPORT
TRUSTED AND SAFE WEBSITE
100% SECURE CHECKOUT
Package Quantity: 1
Results Timing: 3 Days
Test Substance: Cheek Cells
Results Indication: Yes / No
Battery: No Battery Used
•This home collection kit has not been FDA cleared or approved;
•This home collection kit has been authorized by FDA under an EUA;
•This home collection kit has been authorized only for the home collection and maintenance of nasal swab specimens as an aid in detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; and
•This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of medical devices under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
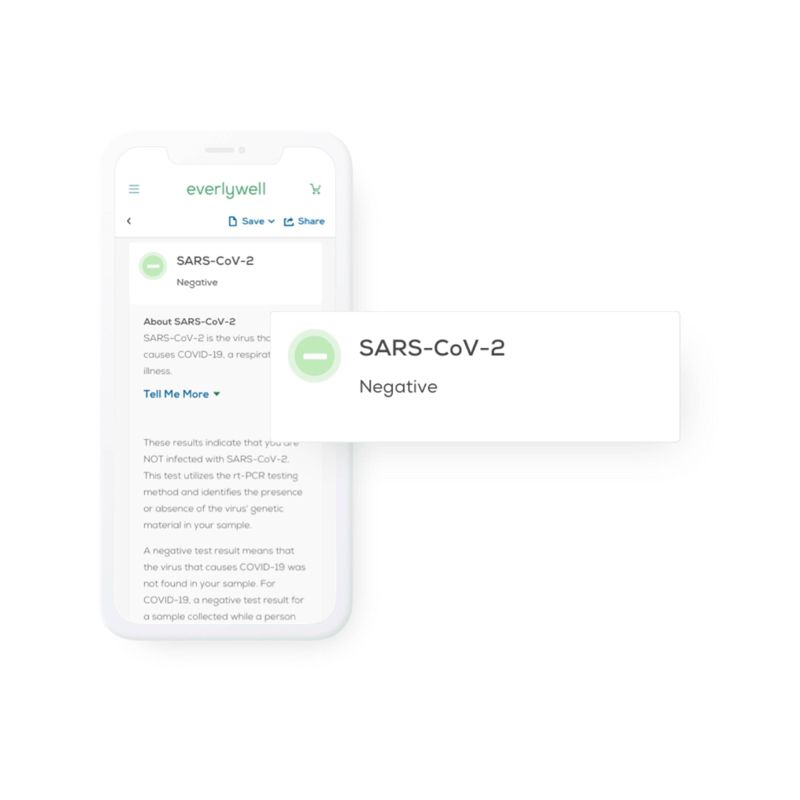
The Everlywell COVID-19 Test Home Collection KIT DTC has received an Emergency Use Authorization (EUA) from the FDA that allows you to conveniently and proactively test for SARS-CoV-2 infection. You collect your own sample at home, ship it to the lab using a prepaid mailer, and receive your secure digital results 24-48 hours after the lab receives your sample. From there, a free telehealth consult is provided to discuss next steps. This collection kit is available to ages 18+ in all 50 states, FSA/HSA-eligible, and may be reimbursed through your health insurance. Everlywell is not enrolled in the Medicare program and this test is ineligible for Medicare reimbursement.
The FDA-authorized Fact Sheet for Individuals comes in the kit and is available online for you to find out more about the Everlywell COVID-19 Test Home Collection Kit DTC.
As a reminder, if you’re experiencing severe symptoms, such as difficulty breathing, seek medical attention right away. The CDC recommends calling your healthcare provider to let them know that you may have COVID-19, which will help them take steps to keep others from getting exposed.
Results Timing: 3 Days
Test Substance: Cheek Cells
Results Indication: Yes / No
Battery: No Battery Used
•This home collection kit has not been FDA cleared or approved;
•This home collection kit has been authorized by FDA under an EUA;
•This home collection kit has been authorized only for the home collection and maintenance of nasal swab specimens as an aid in detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; and
•This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of medical devices under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
The Everlywell COVID-19 Test Home Collection KIT DTC has received an Emergency Use Authorization (EUA) from the FDA that allows you to conveniently and proactively test for SARS-CoV-2 infection. You collect your own sample at home, ship it to the lab using a prepaid mailer, and receive your secure digital results 24-48 hours after the lab receives your sample. From there, a free telehealth consult is provided to discuss next steps. This collection kit is available to ages 18+ in all 50 states, FSA/HSA-eligible, and may be reimbursed through your health insurance. Everlywell is not enrolled in the Medicare program and this test is ineligible for Medicare reimbursement.
The FDA-authorized Fact Sheet for Individuals comes in the kit and is available online for you to find out more about the Everlywell COVID-19 Test Home Collection Kit DTC.
As a reminder, if you’re experiencing severe symptoms, such as difficulty breathing, seek medical attention right away. The CDC recommends calling your healthcare provider to let them know that you may have COVID-19, which will help them take steps to keep others from getting exposed.